本帖最后由 老马 于 2012-11-8 02:16 编辑
# I1 `: |8 K. c( r% ^& W
i' c1 X, K+ p, ]% J, }英文名 BEZ235 Tosylate: p3 X! D. r. t3 k
别名 4-[2,3-Dihydro-3-methyl-2-oxo-8-(3-quinolinyl)-1H-imidazo[4,5-c]quinolin-1-yl]-alpha,alpha-dimethylbenzeneacetonitrile 4-methylbenzenesulfonate* R3 j) d1 Z) `; m" [ ^5 B
产品名称 BEZ235 对甲苯磺酸盐
/ `* `; E f; S+ v1 AFormula:* v& t6 T# _' W5 i* ]' i9 a
C30H23N5O
1 Z* m* z1 G7 u7 r5 BMolecular Weight:
' h/ u0 O3 L/ ~0 E" T1 T. U7 V F469.54' N) J" T3 V% K2 E% p$ r
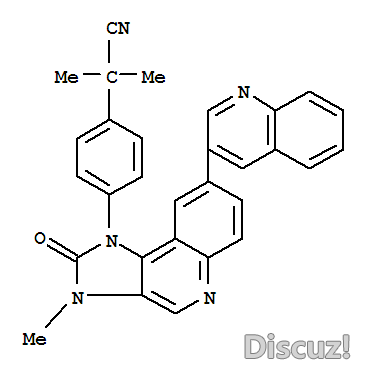
8 D7 A3 s7 k& T! \2-甲基-2-[4-[3-甲基-2-氧代-8-(喹啉-3-基)-2,3-二氢咪唑并[4,5-c]喹啉-1-基]苯基]丙腈
* h* ]9 ^" M3 l2 L7 p
 ; _; G& m# ?- p- D6 z* ~+ \+ j
; _; G& m# ?- p- D6 z* ~+ \+ j
- X2 }/ P: i( ~0 D7 u, x
First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients (pts) with advanced solid tumors.( F8 o: h: i: {( t6 g1 E
Background: The PI3K pathway plays a major role in cancer cell growth and survival, and is frequently aberrantly hyperactivated in tumors. BEZ235 is a potent and highly selective reversible PI3K pathway inhibitor with antiproliferative and apoptotic activity in cancer cells. BEZ235 strongly inhibited activation of downstream effectors (e.g. Akt) and displayed antitumor activity in xenograft models harboring PI3K pathway alterations. In preclinical toxicology studies, BEZ235 treatment was well tolerated. Methods: This phase I, multicenter, open-label, single-agent, dose-escalation, study was conducted in pts with histologically confirmed, advanced, unresectable solid tumors. BEZ235 was administered once daily as a gelatin capsule either fasted or fed (10-1,100 mg) until unacceptable toxicity or disease progression. Dose escalation was guided by a Bayesian logistic regression model with overdose control. Anti-tumor activity of BEZ235 was assessed by CT and PET. Early efficacy data are based on central review. Results: A total of 59 pts have entered the trial (13 breast [22%], 10 CRC [17%]). There were no DLTs with BEZ235 treatment. Frequently reported AEs included nausea, vomiting, diarrhea, fatigue/asthenia, anemia, and anorexia; they were mild or moderate, manageable, and reversible upon treatment discontinuation. AUC and Cmax increased non-proportionally with dose and were variable within and among pts. BEZ235 exhibited dose- and day-dependent PI3K inhibition as measured by elevation of plasma C-peptide levels. 2 PR (1 Cowden syndrome pt, 1 breast pt) and 16 MR were observed. 14 of 51 evaluable pts had stable disease ≥4 months; tumors from 6 of these 14 pts carried dysregulations of the PI3K pathway. 4 of the 14 (29%) patients with SD ≥4 months had breast cancer. 18 of 35 evaluable pts had detectable decreases of 18FDG- uptake. Conclusions: BEZ235 was well tolerated with a favorable safety profile. Available PD and efficacy data show that BEZ235 is active in pts (especially in those with PI3K pathway dysregulated tumors). Based on PK data, future studies will use a new formulation of BEZ235 with improved bioavailability and PK properties. |